







Home > Research > Research Overview > 2024 > Linking Shape, Size, and Function in Complex and Nanomaterial Chemistry
Linking Shape, Size, and Function in Complex and Nanomaterial Chemistry
Associate professor (Nanomaterial Chemistry, Inorganic Chemistry)
ISHIZAKI Manabu
There is a demand for the development of materials and the construction of devices aimed at building a clean energy cycle. To achieve this, techniques and knowledge from complex chemistry and nanomaterial chemistry are combined to control the shape, size, and structure of materials, leading to the development of functions. The dispersion of functional nanomaterials is a key focus for the expression and control of functions.
As representative achievements, Research (1) includes the creation of novel electrodes through the combination of porous coordination polymer nanocrystals and carbon nanotubes for ultra-fast charging and discharging, and Research (2) involves the development of water electrolysis catalysts using nanoparticles controlled at the atomic level of metal composition. In Research (1), the use of zinc metal for the anode led to the successful development of a safe secondary battery that can be charged and discharged in less than 10 seconds, introducing an innovative battery technology. Research (2) achieved the energy-efficient decomposition of water to produce oxygen, aiming to create zinc-air secondary batteries. The goal is to establish technologies that can be returned to society, with ongoing daily research efforts.
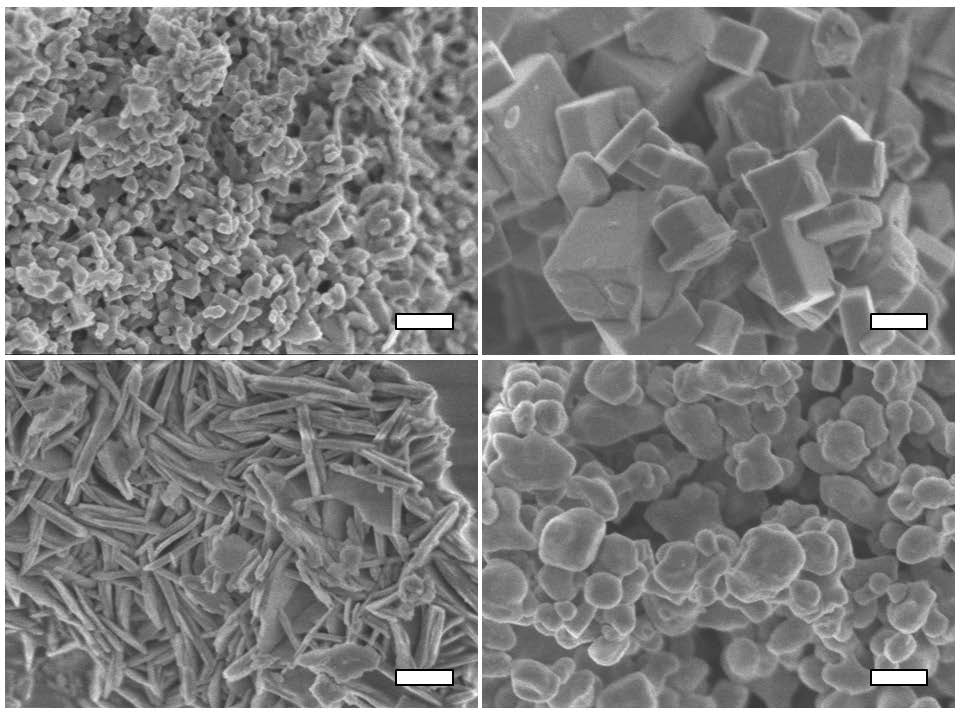
▲Scanning electron microscope images of porous coordination polymer nanocrystals. The scale bars are 100 nm.

▲Photographs of the dispersion solution of porous coordination polymer nanocrystals.
▲The research results on secondary battery development are featured as the back cover illustration of the Journal of Materials Chemistry A, Vol. 11, Issue 48.
Related Links